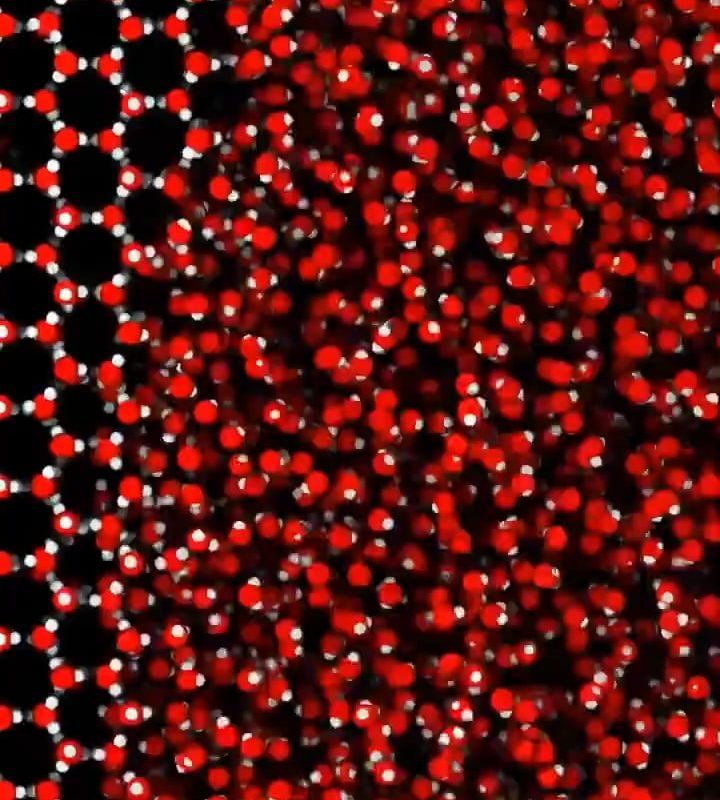
The transformation of water into ice visualized on a molecular level
Title: From Liquid to Solid: The Molecular Dance of Water Freezing into Ice
Meta Description: Discover how water transforms into ice at the molecular level. Explore the science behind hydrogen bonding, crystal formation, and why ice floats. Perfect for science enthusiasts and educators!
Introduction
Every winter, lakes freeze, and ice cubes clink in our drinks, yet few of us pause to consider the invisible molecular ballet that makes this transformation possible. The shift from liquid water to solid ice isn’t just a simple temperature drop—it’s a stunning reorganization of molecules governed by physics and chemistry. In this article, we’ll dive into the microscopic world of water molecules to uncover how this everyday phenomenon unfolds.
The Molecular Structure of Liquid Water
To understand freezing, we first need to examine liquid water. In its liquid state, water molecules (H₂O) are in constant motion, connected by hydrogen bonds—weak attractions between the positively charged hydrogen atoms of one molecule and the negatively charged oxygen atoms of another. These bonds constantly break and reform, allowing water to flow freely while maintaining cohesion.
- Thermal energy: At room temperature, molecules move too rapidly to lock into a fixed arrangement.
- Random arrangement: Liquid water lacks long-range order, resembling a dynamic, tangled network.
The Cooling Process: Slowing Down the Dance
When water cools below 4°C (39°F), its density increases until it reaches the freezing point (0°C or 32°F). As temperatures drop further:
- Kinetic energy decreases: Molecules slow down, reducing their ability to escape hydrogen bonds.
- Bonds stabilize: Hydrogen bonds last longer, encouraging molecules to align into a structured framework.
Nucleation: The Birth of Ice Crystals
Freezing begins with nucleation: the formation of tiny “seed” crystals. This can occur in two ways:
- Homogeneous nucleation: Pure water forms crystals spontaneously at extremely low temperatures (-40°C).
- Heterogeneous nucleation: Impurities (like dust or minerals) or rough surfaces act as anchor points, triggering crystal growth at higher temperatures.
At this stage, water molecules start arranging into a hexagonal lattice, the signature structure of ice.
Crystal Growth: Building the Hexagonal Lattice
Once nucleation occurs, ice crystals grow rapidly as molecules align into a six-sided (hexagonal) pattern. Here’s what happens:
- Order over chaos: Molecules lock into place, with each oxygen atom bonded to four hydrogen atoms (two covalent, two hydrogen bonds).
- Expansion: Unlike most substances, water expands as it freezes. The rigid lattice creates empty space between molecules, making ice less dense than liquid water—which is why icebergs float!
🔬 Visualization Insight: Advanced imaging techniques, like X-ray crystallography, reveal ice’s molecular architecture as a repeating honeycomb-like grid.
Why Does Ice Float? The Anomaly That Sustains Life
Water’s density anomaly is critical for ecosystems. If ice sank, lakes would freeze from the bottom up, destroying aquatic habitats. Instead, the floating ice layer insulates liquid water below, enabling life to thrive in frigid climates.
Real-World Implications
Understanding ice formation has applications beyond curiosity:
- Climate science: Ice sheet dynamics influence sea levels.
- Cryopreservation: Safely freezing biological samples relies on controlling crystal growth.
- Materials engineering: Anti-icing coatings mimic nature to prevent frost damage.
FAQ: Quick Science Bites
Q: Can water exist as a liquid below 0°C?
A: Yes! Supercooling occurs when extremely pure water remains liquid past its freezing point until disturbed.
Q: Why are snowflakes always six-sided?
A: Their shape reflects the hexagonal symmetry of ice’s molecular lattice.
Q: How do impurities affect freezing?
A: Salt or dirt lowers water’s freezing point (used in de-icing roads) and speeds up nucleation.
Conclusion
The transformation of water into ice—a process we often take for granted—is a marvel of molecular coordination. From the frantic motion of liquid molecules to the serene geometry of ice crystals, this phase change illustrates nature’s ability to blend chaos with order. The next time you sip a cold drink or crunch through snow, remember: there’s a hidden universe of physics in every frozen drop.
Keywords for SEO: Water to ice molecular level, freezing process science, hydrogen bonding, ice crystal formation, why does ice float, nucleation in freezing, phase change of water.
📌 Share the Wonder: Tag a science lover or educator to spread the beauty of molecular science! 🌊❄️